

-
৳319.00
৳350.00 -
৳650.00
-
৳660.00
৳900.00
Reviews & Ratings
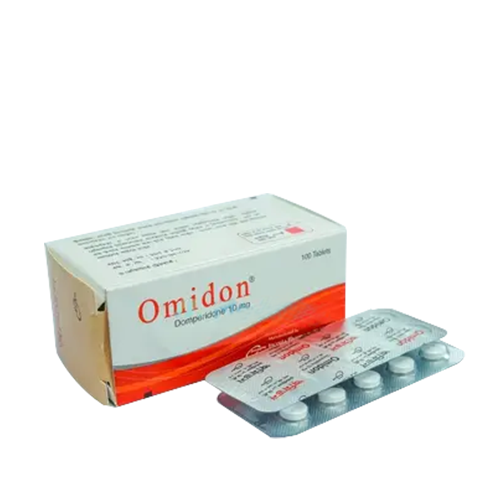
Omidon 10 mg~ 1 Strip
- Tablet-Domperidone Maleate
- Manufacturer: Incepta Pharmaceuticals Ltd
Indications of Omidon 10 mg
Dyspeptic
symptom complex, often linked with delayed gastric emptying, gastroesophageal
reflux, and esophagitis, may include:
·
Epigastric sensation of fullness
·
Abdominal distension
·
Upper abdominal pain
·
Belching
·
Flatulence
·
Early satiety
·
Nausea and vomiting
·
Heartburn, with or without
regurgitation of gastric contents into the mouth
These
symptoms can occur in various conditions, such as non-ulcer dyspepsia, acute
nausea and vomiting of functional, organic, infectious, dietetic origin, or
induced by radiotherapy, drug therapy, or migraine. Additionally, in
Parkinson's disease, it can manifest as dopamine-agonist induced nausea and
vomiting. For radiological studies, it can be used to speed up barium transit
in follow-through radiological examinations.
Theropeutic Class
Motility
Stimulants, Motility stimulants/Dopamine antagonist, Prokinetic drugs
Pharmacology
Omidon
10 mg functions as a dopamine receptor antagonist. It exerts gastroprokinetic
action by inhibiting dopamine receptors found in the chemoreceptor trigger zone
(CTZ) and the stomach. Its limited ability to cross the blood-brain barrier
means it has minimal impact on dopaminergic receptors in the brain, thus
avoiding psychotropic and neurological side effects.
Dosage & Administration of Omidon 10 mg
For
adults, the recommended dosage is 10-20 mg every 4-8 hours daily. Children
should take 0.2-0.4 mg/kg every 4-8 hours daily. When used for acute nausea and
vomiting, the maximum treatment duration should not exceed 12 weeks. It is
advisable to take this medication 15-30 minutes before a meal.
Dosage of Omidon 10 mg
Omidon
10 mg should be taken 15-30 minutes before meals, and if needed, before
bedtime. The typical recommended oral dosage of Domperidone is as follows:
For
Adults:
·
10-20 mg (1-2 tablets or 10-20 ml
suspension) every 6-8 hours daily.
·
The maximum daily dose of
Domperidone is 80 mg.
For
Children:
·
2-4 ml of suspension per 10 kg of
body weight or 0.4-0.8 ml of pediatric drops per 10 kg of body weight, every
6-8 hours daily.
In
cases of dyspeptic symptoms: For Adults:
·
10-20 mg (1-2 tablets or 10-20 ml
suspension) every 6-8 hours daily.
For
Children:
·
0.2-0.4 mg/kg (2-4 ml of suspension
per 10 kg or 0.4-0.8 ml of pediatric drops per 10 kg of body weight) every 6-8
hours daily.
For
acute and sub-acute conditions, especially in cases of acute nausea and
vomiting: For Adults:
·
20 mg (2 tablets or 20 ml
suspension) every 6-8 hours daily.
For
Children:
·
0.2-0.4 mg/kg (2-4 ml of suspension
per 10 kg or 0.4-0.8 ml of pediatric drops per 10 kg of body weight) every 6-8
hours daily.
When
administered rectally in the form of suppositories: For Adults (including
elderly):
·
30-60 mg every 4-8 hours.
For
Children:
·
The maximum daily rectal dose for
children weighing between 10 to 25 kg is 30 mg. The dose may be divided
throughout the day if necessary.
Please
note that the maximum treatment period for acute nausea and vomiting is 12
weeks.
Interaction of Omidon 10 mg
The
simultaneous use of anticholinergic drugs may counteract the antidyspeptic
effects of domperidone. It's important to note that antacids and antisecretory
drugs should not be administered concurrently with domperidone as they can
reduce its oral bioavailability. Domperidone primarily undergoes metabolism
through the CYP3A4 enzyme. In vitro data suggest that coadministration with
drugs significantly inhibiting this enzyme may lead to elevated plasma levels
of domperidone. Examples of CYP3A4 inhibitors include azole antifungals,
macrolide antibiotics, HIV protease inhibitors, nefazodone, and others.
Theoretically,
as domperidone has gastro-kinetic effects, it could potentially impact the
absorption of concurrently orally administered drugs, especially those with
sustained release or enteric-coated formulations. However, in patients already
stabilized on medications like digoxin or paracetamol, the concurrent use of
domperidone did not affect the blood levels of these drugs. Domperidone does
not potentiate the action of neuroleptics, and it suppresses the unwanted
peripheral effects such as digestive disorders, nausea, and vomiting caused by
dopaminergic agonists like bromocriptine and L-dopa without counteracting their
central properties.
Contraindications
Omidon
10 mg is not to be used by patients with a known hypersensitivity to this
medication or in the case of neonates. It should also be avoided in situations
where gastrointestinal stimulation could be unsafe, such as in cases of
gastrointestinal hemorrhage, mechanical obstruction, or perforation.
Additionally, it is contraindicated in patients with a prolactin-releasing
pituitary tumor (prolactinoma).
Side Effects of Omidon 10 mg
Side
effects are uncommon, with occasional reports of transient intestinal cramps.
Extrapyramidal symptoms are rare among young children and exceptionally rare in
adults. These symptoms typically resolve spontaneously and completely upon
discontinuing the treatment. Since the pituitary gland is situated outside the
blood-brain barrier, domperidone may lead to an increase in plasma prolactin
levels. In rare cases, this hyperprolactinemia can result in
neuroendocrinological effects like galactorrhea and gynecomastia. In situations
where the blood-brain barrier is immature (as in infants) or compromised, the
possibility of neurological side effects cannot be entirely ruled out.
Additionally, there have been occasional reports of rare allergic reactions,
including rash and urticaria.
Pregnancy & Lactation
The
safety of using Omidon 10 mg during pregnancy has not been established; hence,
its use is not recommended during pregnancy. Animal studies have not shown any
teratogenic effects on the fetus. Domperidone may induce galactorrhea and
enhance post-natal lactation. It is excreted in breast milk in minimal
quantities, which are insufficient to be considered harmful.
Precautions & Warnings
Omidon
10 mg should be employed with utmost care in children due to the potential for
an increased risk of extrapyramidal reactions, which can be more pronounced in
young children with an incompletely developed blood-brain barrier.
Additionally, considering that domperidone undergoes extensive metabolism in
the liver, it should also be used cautiously in patients with hepatic
impairment.
Overdose Effects of Omidon 10 mg
Signs
of an overdose can manifest as drowsiness, disorientation, and extrapyramidal
reactions, particularly in children. If an overdose occurs, it is advisable to
administer activated charcoal and closely monitor the patient. The use of
anticholinergic drugs, antiparkinson drugs, or antihistamines with
anticholinergic properties may be beneficial in managing extrapyramidal
reactions.
Storage Conditions
Store
below 30°C, Protected from light & moisture. Keep out of children's reach.
Drug Classes
Motility
Stimulants, Motility stimulants/Dopamine antagonist, Prokinetic drugs
Mode Of Action
Omidon
10 mg acts as a dopamine antagonist, primarily by blocking dopamine receptors
located in the Chemoreceptor Trigger Zone (CTZ) and the stomach. Its
gastroprokinetic effect is achieved by inhibiting dopamine receptors that
influence the motility of the gastrointestinal tract. Because it has limited
ability to penetrate the blood-brain barrier, Domperidone has minimal impact on
dopaminergic receptors in the brain, thus avoiding psychotropic and
neurological side effects. Domperidone restores normal motility and tone in the
upper gastrointestinal tract, promotes gastric emptying, enhances antral and
duodenal peristalsis, and regulates pylorus contraction. Additionally, it
increases esophageal peristalsis and lowers esophageal sphincter pressure,
preventing the regurgitation of gastric contents.
Pediatric Uses
Use
in infants: Given that the metabolic and blood-brain barrier functions are not
fully developed during the first months of life, any medication should be
administered to infants with great caution and under close medical supervision.
Although the typical absence of neurological side effects with domperidone is
primarily due to its limited penetration through the blood-brain barrier, the
potential occurrence of such effects cannot be entirely ruled out in infants
under 1 year of age.
Use
in liver disorders: Because domperidone undergoes extensive metabolism in the
liver, it should be employed cautiously in patients with hepatic impairment.
Use
in kidney disorders: In individuals with severe renal insufficiency (serum
creatinine >6 mg/100ml, i.e., >0.6 mmol/l), the elimination half-life of
domperidone increases from 7.4 to 20.8 hours, although plasma drug levels
remain lower than in healthy volunteers. Since very little unchanged drug is
excreted through the kidneys, it is unlikely that the dose of a single acute
administration needs to be adjusted in patients with renal insufficiency.
However, for repeated administration, the dosing frequency should be reduced to
once or twice daily, depending on the severity of the impairment, and the dose
may need to be lowered. In general, patients on prolonged therapy should
undergo regular reviews.
Disclaimer
The
information provided is accurate to our best practices, but it does not replace
professional medical advice. We cannot guarantee its completeness or accuracy.
The absence of specific information about a drug should not be seen as an
endorsement. We are not responsible for any consequences resulting from this
information, so consult a healthcare professional for any concerns or
questions.
Frequently Brought Products
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳319.00
৳350.00 -
৳650.00
-
৳660.00
৳900.00