

-
৳319.00
৳350.00 -
৳650.00
-
৳660.00
৳900.00
Reviews & Ratings
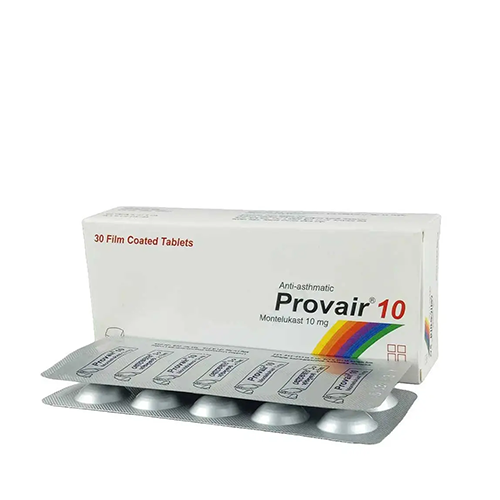
Provair 10 mg~ 1 Strip
- Tablet Montelukast
- Manufacturer: Unimed Unihealth MFG. Ltd.
Indications of Provair 10 mg
Provair 10 mg is indicated for:
·
Prophylaxis
and chronic treatment of asthma
·
Acute
prevention of Exercise-Induced Bronchoconstriction (EIB)
·
Relief
of symptoms of Allergic Rhinitis (AR): Seasonal & Perennial Allergic
Rhinitis
Theropeutic Class
Antibodies to leukotriene receptors
Pharmacology
The arachidonic acid metabolism results in the
production of cysteinyl leukotrienes (LTC4, LTD4, and LTE4) from a variety of
cells, including mast cells and eosinophils. Cysteinyl leukotriene receptors
(CysLT) are located in the human airway, and these eicosanoids bind to them.
The orally active drug Provair 10 mg has a strong affinity and specificity for
the CysLT1 receptor. Without any agonist activity, it inhibits LTD4's
physiological effects at the CysLT1 receptor.
Dosage & Administration of Provair 10 mg
Adults and adolescents with
asthma or seasonal allergic rhinitis:
·
The dosage for adults and adolescents 15 years of age and older: Monas 10 mg tablet
once daily.
Pediatric
patients with asthma or seasonal allergic rhinitis:
·
The dosage for pediatric patients 6 to 14 years of age: Monas 5 mg tablet
once daily.
·
The dosage for pediatric patients 2 years to 5 years of age: Monas 4 mg tablet
once daily.
·
The dosage for pediatric patients 6 months to 5 years of age: Monas 4 mg oral granules
once daily. This can be administered either directly in the mouth, or mixed
with a spoonful of cold water or soft food at room temperature
Use in the pediatric patient: The
safety and efficacy of Monas have been established in adequate and well-controlled
studies in pediatric patients with asthma 6 months to 14 years of age. Safety
and efficacy profiles in this age group are similar to those seen in adults.
Hepatic
Insufficiency: No dosage adjustment is required in patients
with mild-to-moderate hepatic insufficiency.
Renal
Insufficiency: No dosage adjustment is recommended in patients
with renal insufficiency.
Elderly
use: The pharmacokinetic profile and the oral bioavailability
of a single 10-mg oral dose of Monas are similar in elderly and younger adults.
The plasma half-life of Monas is slightly longer in the elderly. No dosage
adjustment in the elderly is required.
Interaction of Provair 10 mg
In the prevention and long-term treatment of
asthma, Provair 10 mg has been provided alongside other commonly used
treatments without seeming to increase the risk of negative side effects.
According to studies on drug interactions, theophylline, prednisone,
prednisolone, oral contraceptives (norethindrone 1mg/ethinyl estradiol 35mcg),
terfenadine, digoxin, and warfarin did not exhibit any clinically significant
effects on the pharmacokinetics at the recommended clinical dose of it. In
clinical investigations, the medication was used concurrently with a variety of
routinely prescribed medications without showing any signs of clinically
harmful interactions, despite the fact that more detailed interaction studies
were not carried out. Thyroid hormones, hypnotic sedatives, benzodiazepines,
non-steroidal anti-inflammatory drugs, and decongestants were among these
drugs. Following a single dose of this solution, the hepatic
metabolism-inducing drug phenobarbital reduced the drug's AUC by about 40%. For
this medication, no dosage modification is advised. When powerful cytochrome
P450 enzyme inducers, such as phenobarbital or rifampin, are provided alongside
this solution, it is fair to use proper clinical monitoring.
Contraindications
Those who have an extreme sensitivity to any
ingredient in this medication should not take it.
Side Effects of Provair 10 mg
Common: upper respiratory tract infection,
diarrhea, fever, gastrointestinal pain, headache, nausea, and vomiting. The
following symptoms are uncommon: akathisia, anxiety, arthralgia, asthenia,
aberrant behavior, depression, dizziness, drowsiness, dry mouth, hemorrhage,
irritability, malaise, muscle complaints, oedema, seizure, odd feeling, and
sleep disturbances. Rare: Pulmonary eosinophilia, angioedema, decreased
attention, disorientation, erythema nodosum, hallucinations, hepatic problems,
memory loss, palpitations, suicidal thoughts, and tremor.
Pregnancy & Lactation
Provair 10 mg is classified as pregnancy
category B. Although it has been demonstrated that the medication crosses the
placenta of pregnant rats and rabbits, there are no records of its use in
pregnant humans. Although there is some evidence that it is also secreted in
breast milk, little is known about the implications of this discovery. Before
starting the therapy in nursing moms, caution should be exercised.
Precautions & Warnings
The reversal of bronchospasm in acute asthma
attacks, including status asthmaticus, is not recommended as usage for Provair
10 mg. Patients should be told to have access to the proper rescue medication.
Asthma exacerbations that are acute can still be treated with the solution.
While the dosage of an inhaled corticosteroid may be gradually decreased under
medical supervision, the use of this solution instead of an inhaled or oral
corticosteroid should not be started suddenly. Exercise-induced bronchospasm should
not be treated and managed with a single dose of it. While taking it, patients
with documented aspirin sensitivity should continue to refrain from taking
aspirin or non-steroidal anti-inflammatory drugs. Although aspirin sensitivity
in asthmatics is a known side effect of the drug, it has not been demonstrated
to shorten the bronchoconstrictor response to aspirin and other non-steroidal
anti-inflammatory drugs (NSAIDs).
Overdose Effects of Provair 10 mg
The majority of overdose reports did not
include any negative experiences. The most frequent side effects, which were in
line with Provair 10 mg's safety profile, were nausea, vomiting, psychomotor
hyperactivity, thirst, headache, and stomach pain. In the case of an overdose,
it is permissible to take the customary supportive measures, such as removing
any unabsorbed material from the digestive tract, using clinical monitoring,
and if necessary, instituting supportive therapy.
Storage Conditions
Store below 30°C in a cool, dry environment
while keeping out light and moisture. Keep out of children's reach.
Drug Classes
Leukotriene receptor antagonists
Mode Of Action
The cysteinyl leukotriene receptor is inhibited
by the selective and orally active leukotriene receptor antagonist known as
Provair 10 mg (CysLT1). The arachidonic acid metabolism results in the
production of cysteinyl leukotrienes (LTC4, LTD4, and LTE4) from a variety of
cells, including mast cells and eosinophils. The pathophysiology of asthma and
allergic rhinitis, including airway edema, smooth muscle contraction, and
altered cellular activity associated with the inflammatory process, have been
linked to cysteinyl leukotrienes and leukotriene receptor occupation. These
factors all contribute to the signs and symptoms of asthma.
Pregnancy
After oral administration, Provair 10 mg passes the placenta in rats and rabbits. Nevertheless, there isn't any good, controlled research on pregnant women. Because the human reaction to medications is not usually predicted by research on animal reproduction, pregnant women should only use it if absolutely necessary. When giving the pill to a nursing mother, care should be taken because many medications are secreted in human milk.
Disclaimer
The information provided is accurate to our
best practices, but it does not replace professional medical advice. We cannot
guarantee its completeness or accuracy. The absence of specific information
about a drug should not be seen as an endorsement. We are not responsible for
any consequences resulting from this information, so consult a healthcare
professional for any concerns or questions.
Frequently Brought Products
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
-
৳319.00
৳350.00 -
৳650.00
-
৳660.00
৳900.00